Background: Patients (pts) with low-risk MF are typically excluded from interventional clinical trials, thus data regarding the prognosis of this pt population are largely derived from retrospective analyses. The Myelofibrosis and Essential Thrombocythemia Observational Study (MOST; NCT02953704) is a prospective observational study that enrolled pts with low-risk MF defined by Dynamic International Prognostic Scoring System (DIPSS) criteria. In this analysis, we report disease progression, defined by worsening of clinical or laboratory parameters, in MF pts during the MOST study.
Methods: Criteria for enrollment in MOST were confirmed locally and centrally, and included a physician-reported diagnosis of MF (PMF, post-PV, or post-ET) and lack of any DIPSS risk factors except for age >65 y. Of the 232 MF pts enrolled, 205 met this criteria upon central review and were included in this analysis as Cohort A; the remaining 27 pts were enrolled despite having ≥1 DIPSS risk factor (other than age >65 y) and were analyzed separately as Cohort B. Data were collected as previously described (Gerds A. CLML. 2022;22:e532). Disease progression on study was defined as the acquisition of 1 of the following criteria: hemoglobin (Hb) <10 g/dL, platelets <100×10 9/L, presence of constitutional symptoms (weight loss, fever, or sweats), new or worsening splenomegaly, blasts >1%, WBC count >25×10 9/L, death due to disease progression, leukemic transformation (LT), or >1 RBC transfusion. Median (range) follow-up was 52.9 months (42-68).
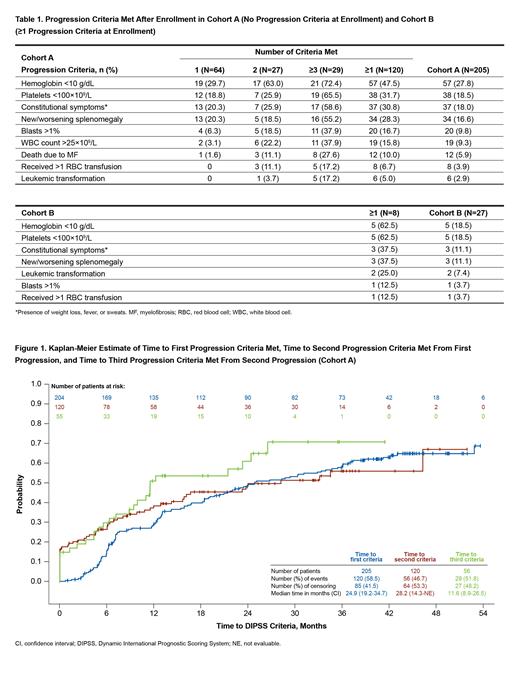
Results: In Cohort A, 120/205 pts (58.5%) had evidence of disease progression during the study. Baseline characteristics comparing pts with vs without disease progression were similar; although compared with pts without progression, those who progressed were older at diagnosis (median [range], 65.9 [21-88] vs 62.9 [35-86] y), older at enrollment (median [range], 70.5 [35-88] vs 66.0 [35-88] y), and had longer median (range) time from diagnosis to enrollment (1.9 [0-38] vs 1.4 [0-24] y). Similar percentages of pts with vs without progression were receiving MF-directed therapy at enrollment (59.2% vs 61.2%); most common treatments were ruxolitinib (33.3% vs 14.1%) and hydroxyurea (22.5% vs 36.5%). Of pts in Cohort A tested for JAK2 mutations, 66/93 (71.0%) with disease progression and 42/65 (64.6%) without were positive. Of pts tested for CALR mutations, 16/21 (76.2%) with disease progression and 17/23 (73.9%) without were positive. Among the 120 pts with progression, 64 (53.3%) met 1 criteria, 27 (22.5%) met 2 criteria, and 29 (24.2%) met ≥3 criteria during the study. The most common progression criteria met were Hb <10 g/dL (47.5%), platelets <100×10 9/L (31.7%), constitutional symptoms (30.8%), and new/worsening splenomegaly (28.3%) (Table 1). Median (95% CI) durations to 1st, 2nd, and 3rd progression criteria were 24.9 (19.2-34.7), 28.2 (14.3-NE), and 11.6 (8.9-26.5) months, respectively (Figure 1). Of pts who progressed, 6 (5.0%) developed LT and 12 (10.0%) died due to disease progression.
In Cohort B at time of enrollment, 25/27 pts (92.6%) met 1 progression criteria. The most frequent was Hb <10 g/dL (13/27 [48.1%]), followed by WBC count >25×10 9/L (7/27 [25.9%]); constitutional symptoms (4/27 [14.8%]), and blasts >1% (1/27 [3.7%]); 1 pt (3.7%) met 2 progression criteria (Hb <10 g/dL and constitutional symptoms), and 1 pt (3.7%) met 3 progression criteria (blasts >1%, constitutional symptoms, and WBC count >25×10 9/L). Eight of 27 pts (29.6%) had evidence of further disease progression while on study (Table 1). The most common new progression criteria met were Hb <10 g/dL and platelets <100×10 9/L (each 5/8 [62.5%]) and constitutional symptoms and new/worsening splenomegaly (each 3/8 [37.5%]).
Conclusions: This real-world observational analysis from MOST is the first prospective analysis of disease progression in low-risk myelofibrosis. The results show that with over 4 y of follow-up, nearly 60% of pts with low-risk MF had evidence of disease progression; 17/205 pts (8.3%) developed LT or died (both occurred in 1 pt). Median time to acquisition of 1st and 2nd disease progression criteria was approximately 2 y, respectively, decreasing to approximately 1 y for the 3rd criteria; this indicates a slow but increasing rate of progression over the course of the study. These data provide real-world insight into disease progression rate in pts with low-risk myelofibrosis.
Disclosures
Grunwald:Karius, Novartis, Ono Pharmaceutical, Pfizer, Pharmacosmos, Premier, Stemline Therapeutics: Consultancy; Incyte Corporation, Janssen: Research Funding; Medtronic: Current equity holder in publicly-traded company; AbbVie, Agios/Servier, Amgen, Astellas Pharma, Blueprint Medicines, Bristol Myers Squibb, Cardinal Health, CTI BioPharma/Sobi, Daiichi Sankyo, Gamida Cell, Genentech, Gilead Sciences, GSK/Sierra Oncology, Incyte Corporation, Invitae, Jazz Pharmaceuticals: Consultancy. Gerds:Accurate Pharmaceuticals, Constellation Pharmaceuticals, CTI BioPharma, Imago BioSciences, Incyte Corporation, Kratos Pharmaceuticals: Research Funding; AbbVie, Bristol Myers Squibb, Constellation Pharmaceuticals, GlaxoSmithKline, Kartos, Novartis, PharmaEssentia, Sierra Oncology: Consultancy. Agrawal:Incyte Corporation: Speakers Bureau. Braunstein:Incyte Corporation: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Hamer-Maansson:Incyte Corporation: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Kalafut:Incyte Corporation: Current Employment, Current equity holder in publicly-traded company. Mascarenhas:Bristol Myers Squibb, Celgene, Constellation Pharmaceuticals/MorphoSys, CTI BioPharma, Galecto, Geron, GSK, Incyte Corporation, Karyopharm Therapeutics, Novartis, PharmaEssentia, Prelude Therapeutics, Pfizer, Merck, Roche, AbbVie, Kartos: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie, Bristol Myers Squibb, Celgene, CTI BioPharma, Geron, Incyte Corporation, Novartis, Janssen, Kartos Therapeutics, Merck, PharmaEssentia, Roche: Research Funding; Bristol Myers Squibb, Celgene, CTI BioPharma, Geron, Incyte Corporation, Janssen, Kartos Therapeutics, Merck, Novartis, PharmaEssentia, Roche; Participated in consulting or advisory committees - AbbVie, Bristol Myers Squibb, Celgene, Constellation Pharmac: Research Funding; Incyte, Novartis, Roche, Geron, GSK, Celgene/BMS, Kartos, AbbVie, Karyopharm, PharmaEssentia, Galecto, Imago, Sierra Oncology, Pfizer, MorphoSys, CTI Bio: Consultancy; GSK: Honoraria; AbbVie, CTI BioPharma Corp, a Sobi company, Geron, GlaxoSmithKline, Imago, Incyte, Kartos, Kayropharm, MorphoSys, Novartis, Pfizer, PharmaEssentia, Sierra: Consultancy.